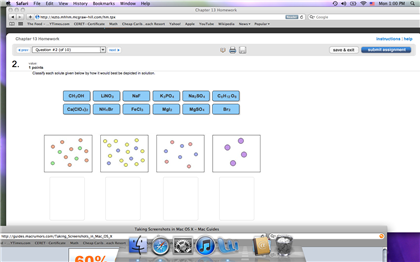
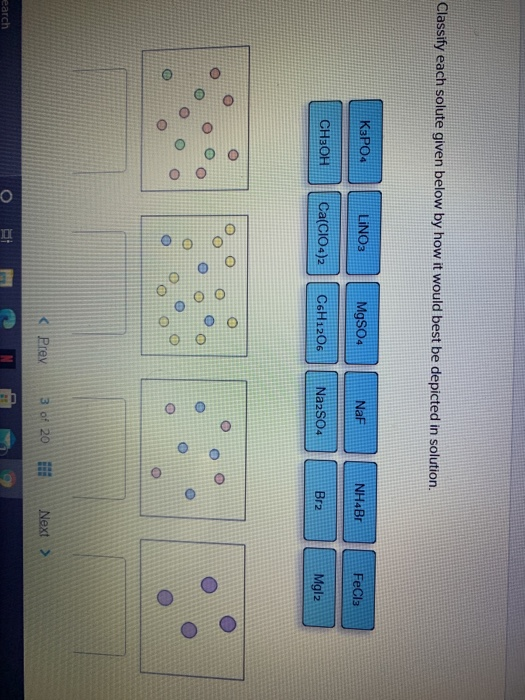
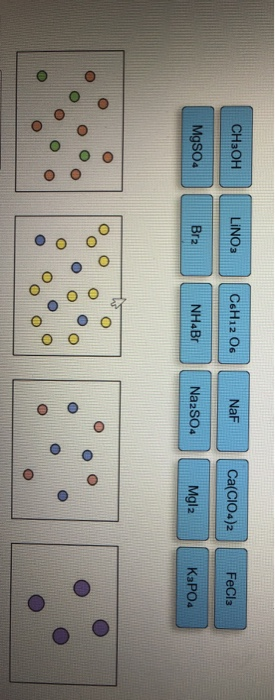
Classify Each Solute Given Below By How It Would Best Be Depicted In Solution.
Classify each solute given below by how it would best be depicted in solution.. A 235010-2 M solution of NaCl in water is at 20 C. Olume of solvent increases the mass of solute that can dissolve in a saturated solution increases creasesstays the same. It was determined that the volume of water is needed to do this was 9994 mL.
The question is Classify each solute given below by how it would best be depicted in solution. The sample was created by dissolving a sample of NaCl in water and then bringing the volume up to 1000 L. Bra Nola Fech LINO.
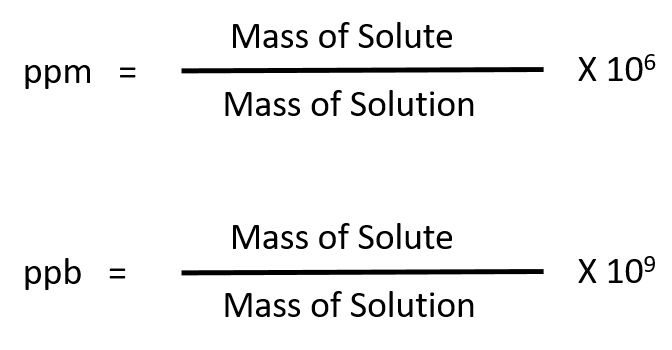
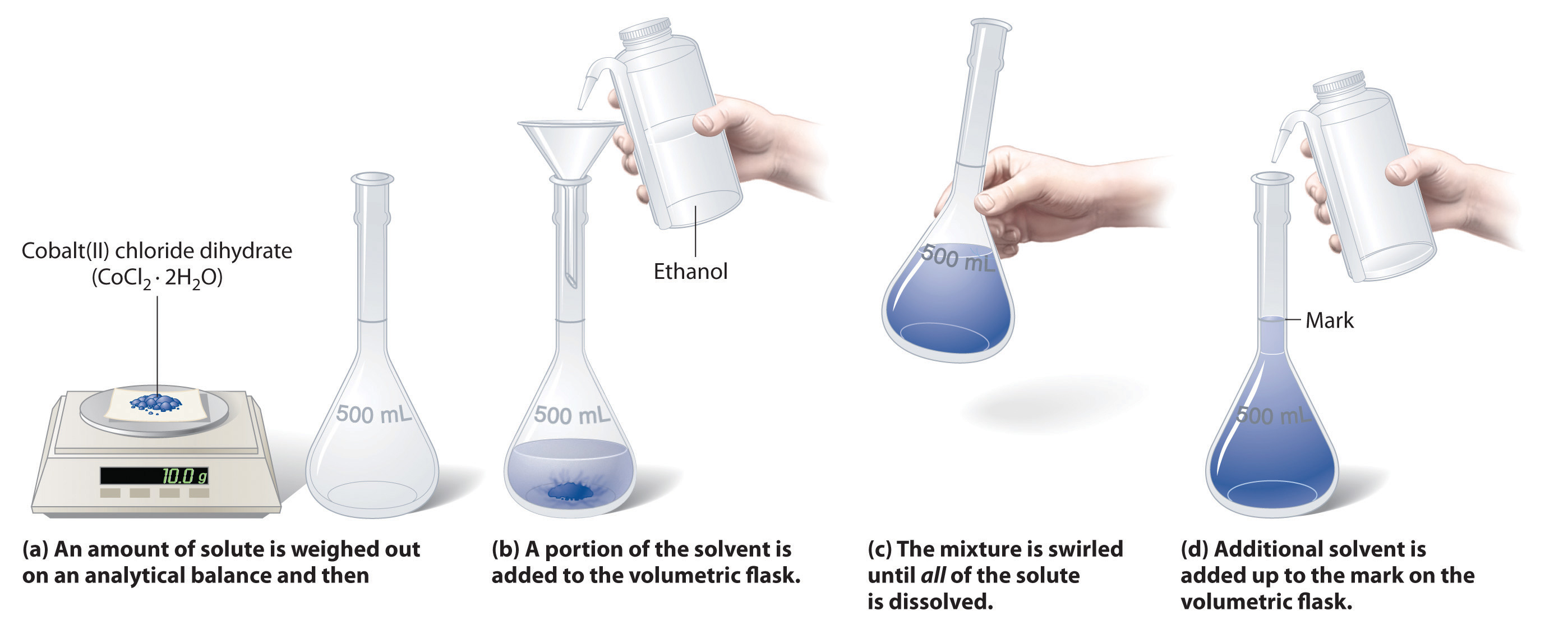
Calculate the concentration of the salt solution in parts per. The images represent a solution of NaNO3 a more concentrated solution of NaNO3 and an MgCl2 solution. If you need to find the mass of the solute then weigh it on a lab scale and record the measurement.
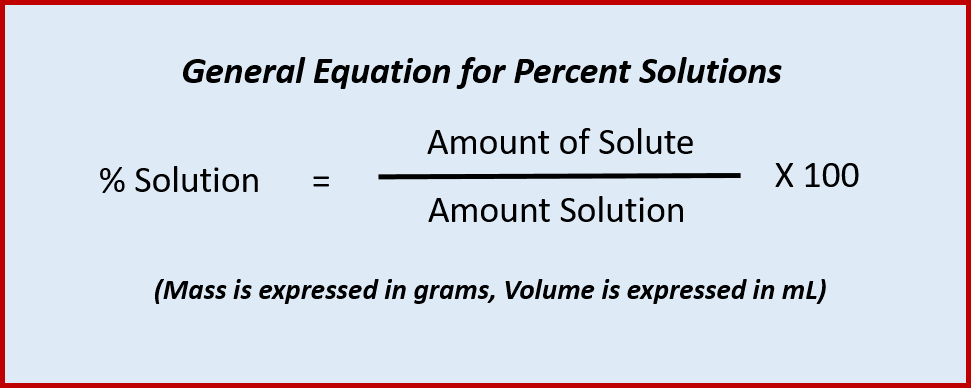
This problem has been solved. Water is an exception. The concentration of a solution is the amount of solute dissolved in a given quantity of solution.
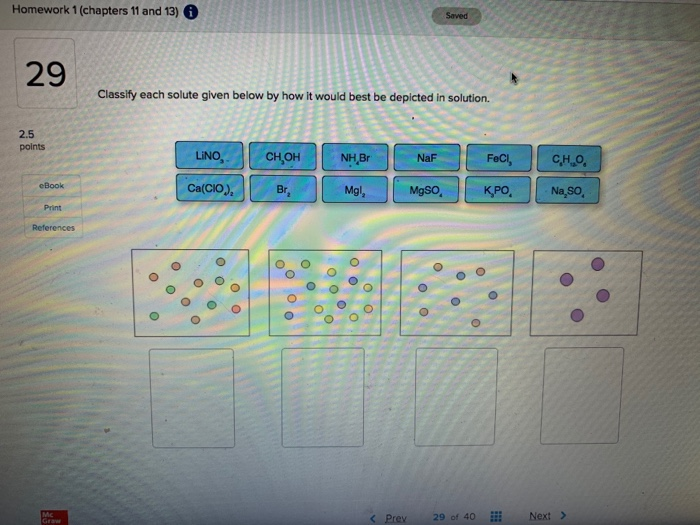
Classify Each Solute Given Below By How It Would Best Be Depicted In Solution. NH Br NaF CHOH LINO Print erences no w Prev 9 of 12 Next. When the volume of solvent increases the solubility of a solute at a given temperature increasesdecrease tays the same.
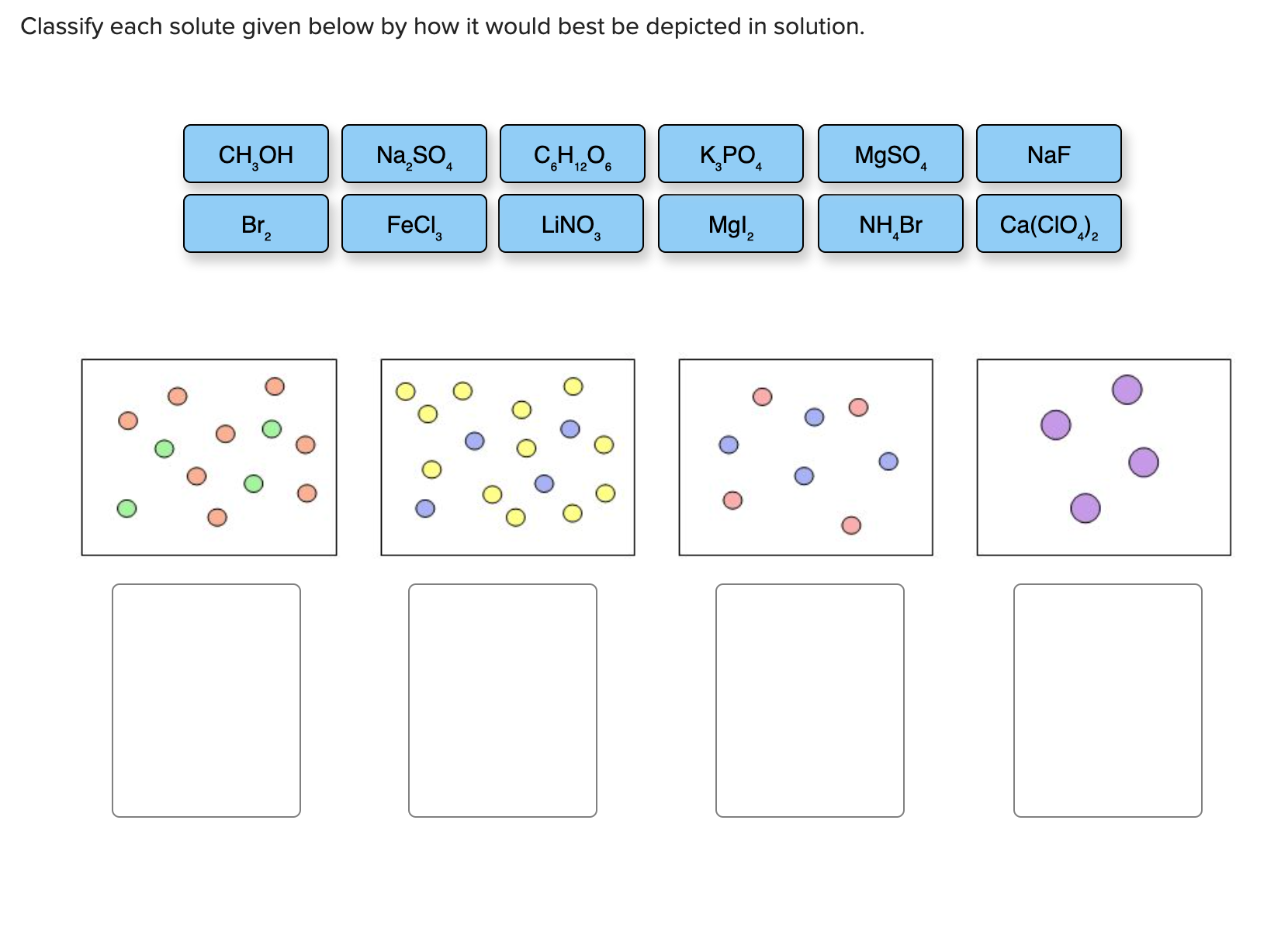
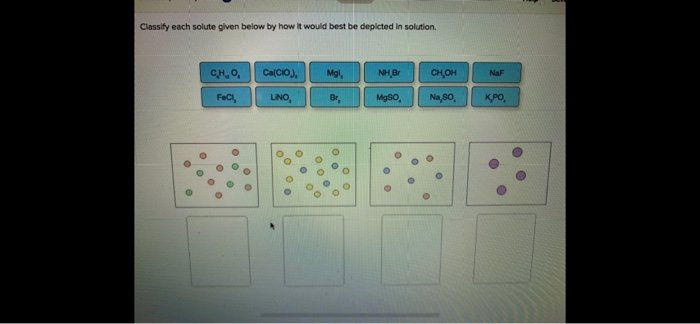
When the solution equilibrium point is reached and no more solute will dissolve the solution is said to be saturated. Classify each solute given below by how it would best be depicted in solution CH OH MgSO. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent.
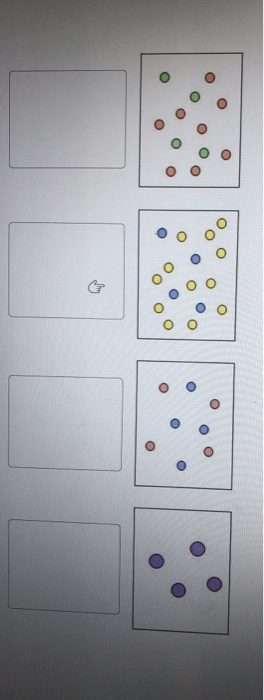
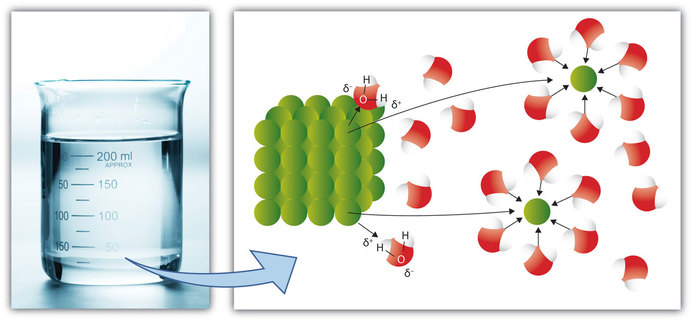
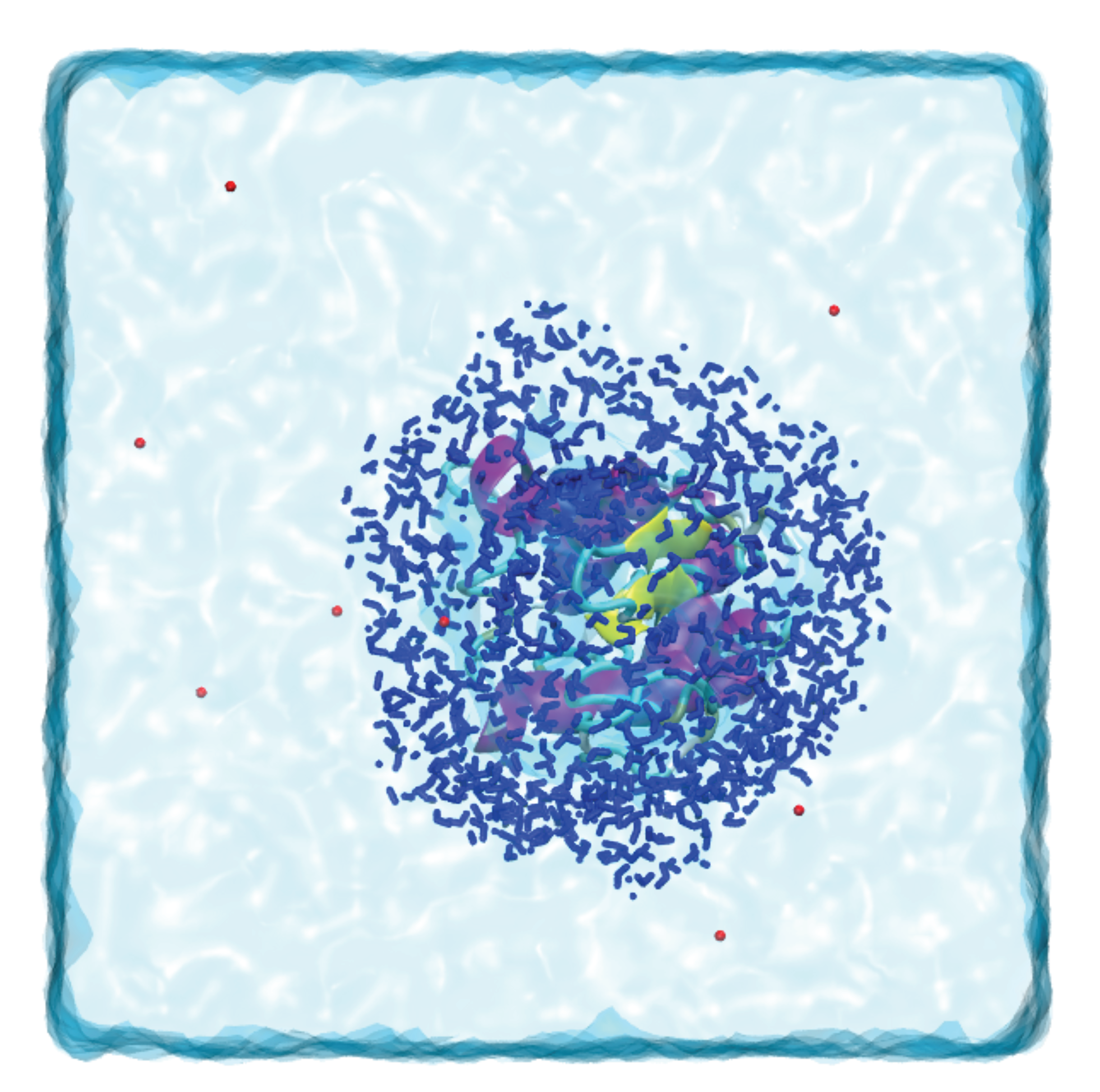
For each solute listed below identify the better solvent for solution formation. Each of the following figures represents an aqueous solution.
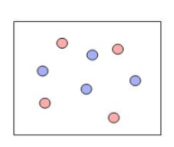
Each of the following figures represents an aqueous solution.
Classify each solute given below by how it would best be depicted in sol. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. When the solution equilibrium point is reached and no more solute will dissolve the solution is said to be saturated. It is always considered as the solvent regardless of amount. The sample was created by dissolving a sample of NaCl in water and then bringing the volume up to 1000 L. In a solution the component present in a smaller proportion is called the solute while the component present in larger proportion is called the solvent. Classify each solute given below by how it would best be depicted in solution. For each solute listed below identify the better solvent for solution formation. Classify each solute given below by how it would best be depicted in solution CH OH MgSO.
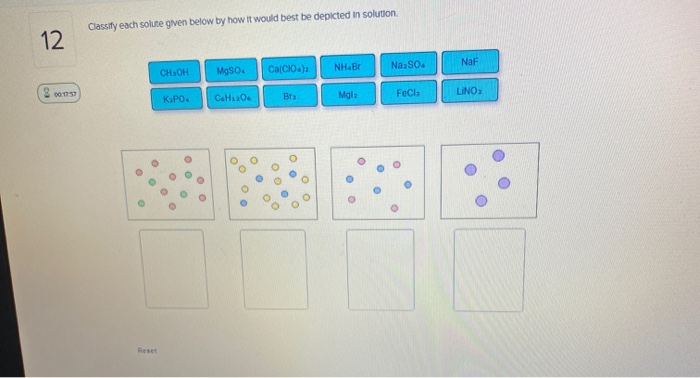
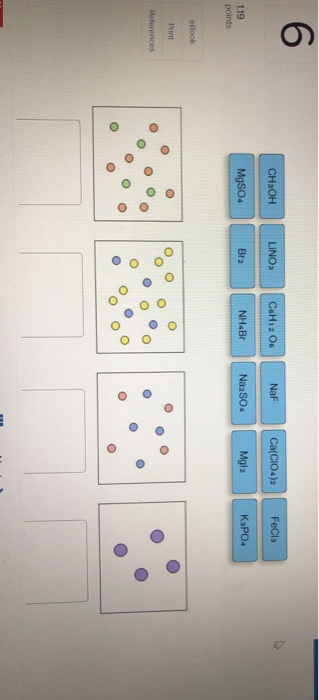
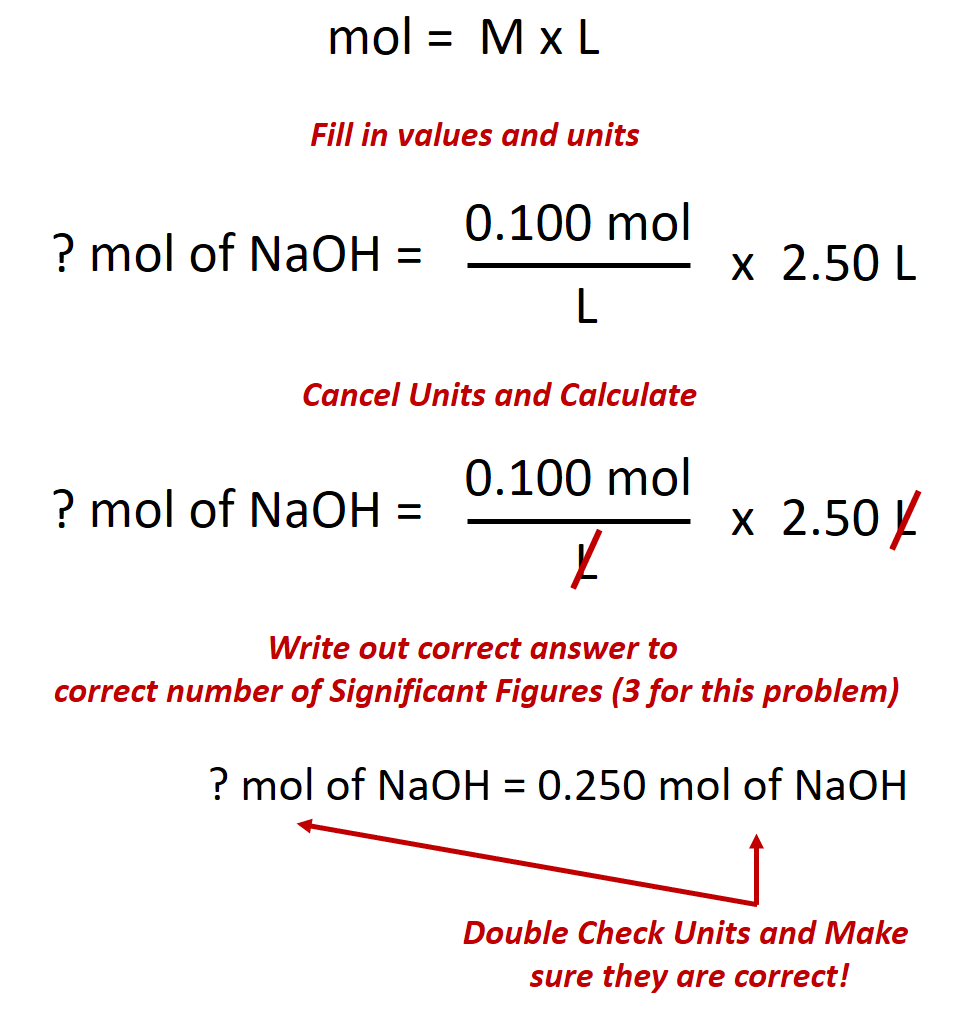
When one substance dissolves into another a solution is formed. For the same solution determine the vant Hoff factor assuming 100 ionization. Classify each solute given below by how it would best be depicted in solution Mgla MgSo. A 235010-2 M solution of NaCl in water is at 20 C. Water is an exception. When the solution equilibrium point is reached and no more solute will dissolve the solution is said to be saturated. The density of water at 20 C is 09982 gmL.






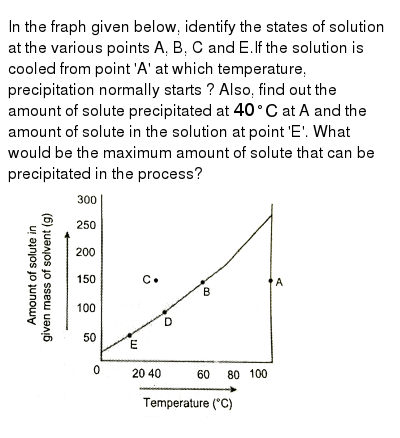


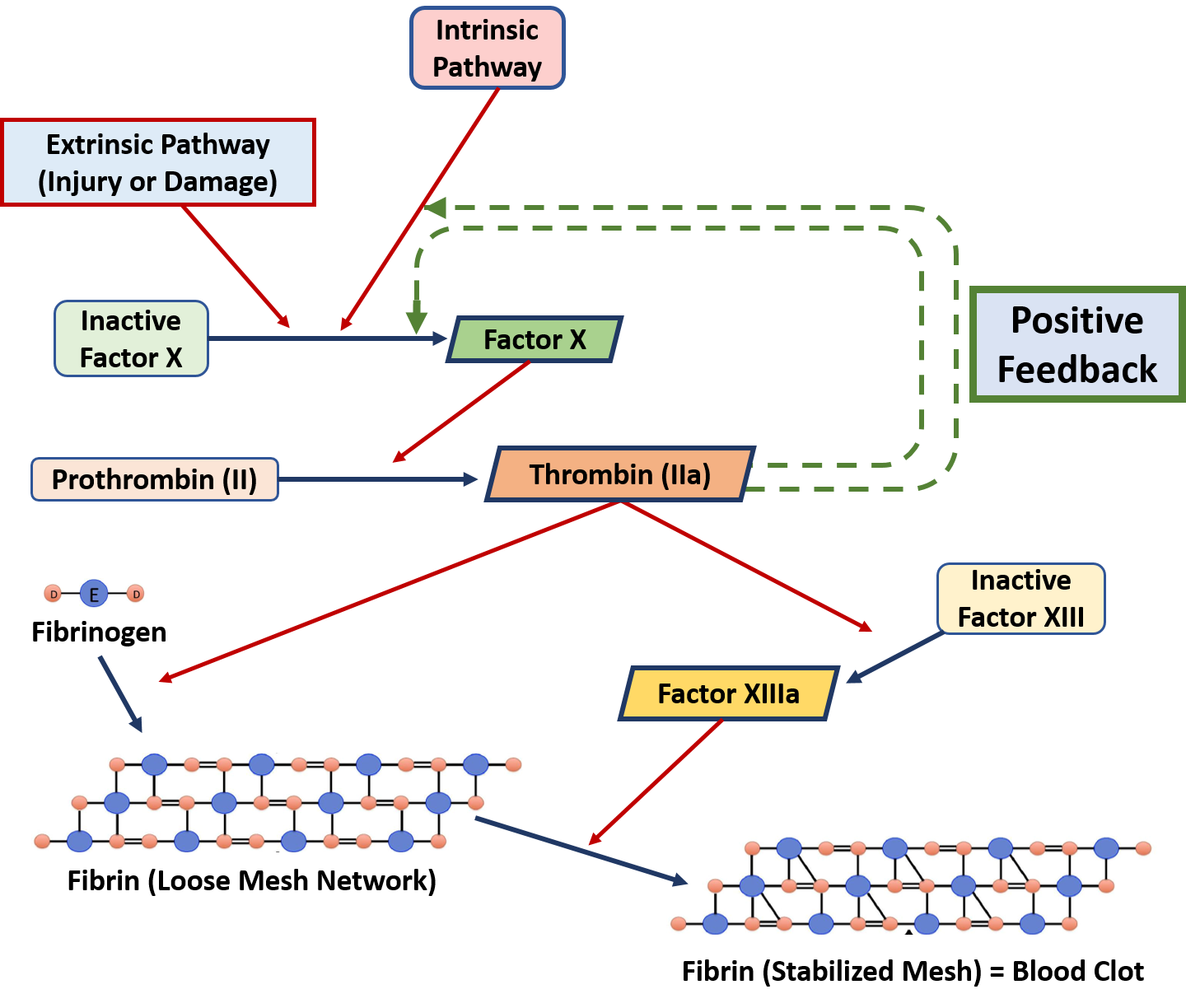

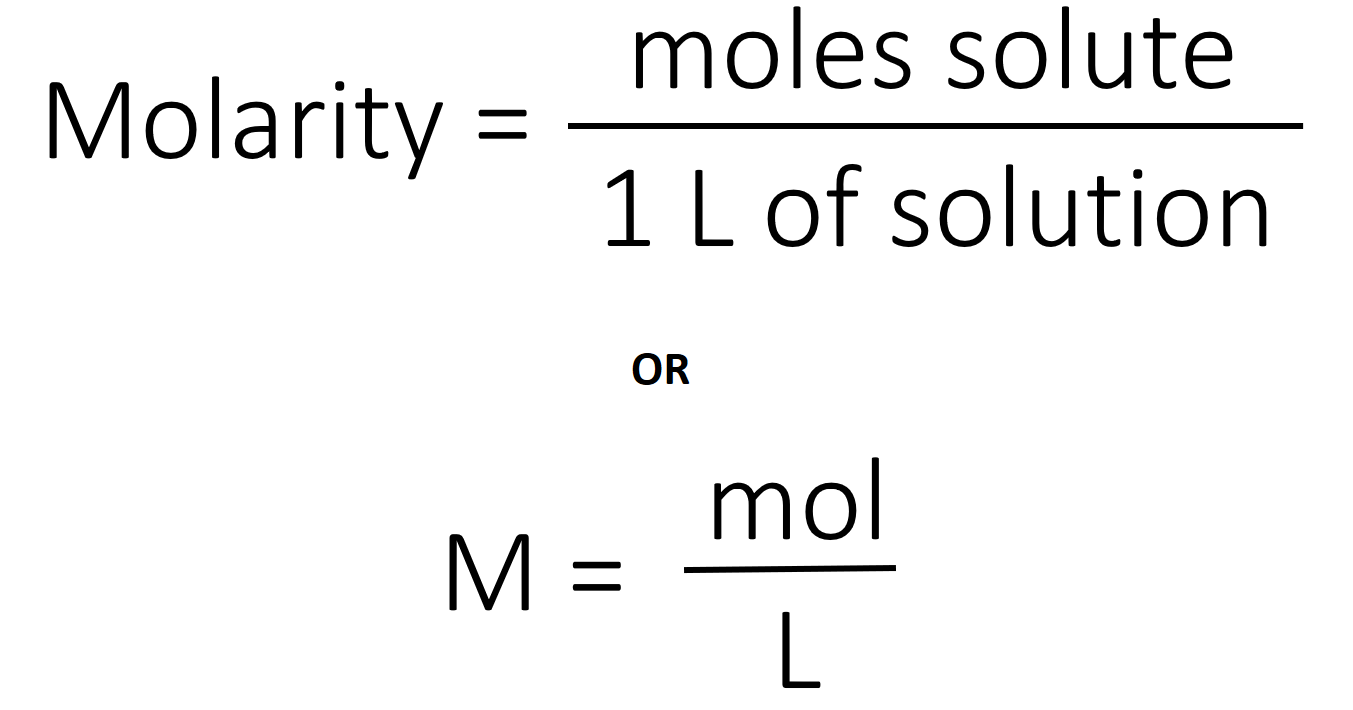






Posting Komentar untuk "Classify Each Solute Given Below By How It Would Best Be Depicted In Solution."